Prescribing thyroid hormones with antidepressants—whether to augment the antidepressant effect or accelerate patient response—is a well-researched strategy for treatment-resistant major depressive disorder (MDD). Thyroid hormones are known to boost response to tricyclics, and preliminary evidence shows they may be useful adjuvants to selective serotonin reuptake inhibitors (SSRIs) as well.
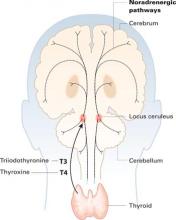
Thyroid hormones enter the brain slowly across the blood-brain barrier and choroid plexus. They accumulate in the locus ceruleus and other structures and are distributed widely along noradrenergic pathways.
Effective treatments are available for MDD, although 30% to 40% of patients do not respond to one or more antidepressant trials (Box).1-4 This article offers:
- new information about why triiodothyronine (T3) and thyroxine (T4) can “super-charge” antidepressant response
- tips on how to use thyroid hormones in patients with MDD, including effective dosages, patient monitoring, and treatment durations.
- 30% to 40% of patients with major depressive disorder (MDD) do not respond sufficiently to usual antidepressant treatment1
- Even under optimal treatment conditions, only one-third of patients achieve remission2
- Among patients who fail to respond to two pharmacologic interventions, remission rates with the next antidepressant are as low as 12%3
- A patient becomes less likely to respond clinically with each additional nonresponse to antidepressant treatment4
Why thyroid hormones?
Thyroid hormones enter the brain slowly across the blood-brain barrier and the choroid plexus—cerebrospinal fluid barriers. T4 is the main source of brain T3—after attack by 5’deiodinase—but circulating T3 also crosses the blood-brain barrier through active transport.
Thyroid hormones accumulate in the locus ceruleus and other central noradrenergic structures and are distributed widely in the brain along noradrenergic pathways.5-7 The mechanism of their therapeutic effect for MDD is not well understood, and various hypotheses have been proposed.
Subclinical hypothyroidism. Early studies such as by Howland8 of treatment-refractory MDD suggested that thyroid hormone augmentation might correct a hypothyroid state. However, blood thyroid hormone levels are not associated with resistance to antidepressant treatment, according to studies of MDD populations.9-10 Also, thyroid hormones’ therapeutic action in MDD appears unlikely to be related to treating subclinical hypothyroidism because patients’ euthyroid status was verified in all adjuvant studies since 1980.
Joffe et al11 proposed that MDD is characterized by a relative excess of T4 versus T3—probably related to a deficit in converting T4 to T3 in the periphery—and administering T3 would therefore correct this imbalance. They offered no strong evidence for an increased T4 level, however, and later studies failed to detect the postulated blood T3 abnormalities in MDD.9-10 Also—as suggested by studies with high-dose T4 augmentation12,13—the adjuvant antidepressant effect of thyroid hormones is not restricted to T3, although T3 may be more efficacious than T4.14
Neurotransmitter effects. Thyroid hormones’ role in increasing serotonin (5-HT) release could partially explain the benefit of adjuvant thyroid hormone therapy in MDD. Researchers found:
- T3 increased cortical 5-HT levels, probably by reducing the autoinhibitory effect of the presynaptic 5-HT1A receptor15
- adding T3 to clomipramine therapy increased 5-HT levels to a greater extent than T3 or clomipramine used alone16
- Low 5-HT activity, shown in hypothyroid patients, increased after T4 replacement.17
This 5-HT release theory cannot explain why thyroid hormones have a rapid clinical effect in MDD. Most studies report the hormones have clinical efficacy in MDD within 4 weeks.18 Similarly, selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels within hours of treatment onset, but the clinical effect occurs 4 to 6 weeks later.19 Therefore, increased 5-HT levels cannot fully explain thyroid hormones’ early effect.
Close interaction between thyroid hormones and the noradrenergic system also has been examined. Brain T3 is primarily localized in the central noradrenergic systems, with axonal anterograde transport of T3 from the locus ceruleus. T3 is processed and accumulated in the noradrenergic system, carried via axonal transport, then delivered from nerve cell bodies to its neuronal targets.5,7 T3 thus functions as a coneurotransmitter with norepinephrine.
Cellular energy metabolism. We recently reported that thyroid hormones’ antidepressant effect may be related to brain cellular energy metabolism. Thyroid hormones increase cellular levels of adenosine triphosphate (ATP) and phosphocreatine (PCr) in the hypothyroid brain.20 Brain imaging—phosphorus-31 nuclear magnetic resonance spectroscopy (31P-MRS)—of subjects with MDD shows decreased brain levels of ATP and increased PCr.21
Our group showed that the antidepressant effect of T3 augmentation of SSRIs is correlated with significant increases in ATP levels and decreases in PCr. This effect—which appears to represent re-normalization of brain bioenergetics in treatment responders—did not occur in nonresponders (Iosifescu et al, presented at APA, 2004).
The effect of thyroid hormones on bioenergetic metabolism is compatible with the hypothesized effects on noradrenergic and serotonergic systems.5,7 These mechanisms may represent different links in the same chain of events.