The less time that passes between the onset of psychosis and initiation of appropriate treatment, the greater the patient’s odds of recovery.1 However, relapse prevention is a major clinical challenge because >80% of patients will relapse within 5 years, and, on average, 40% to 50% of patients with a first-episode schizophrenia will relapse within 2 years depending on the definition used and patient characteristics.2 Although there are several explanations and contributing factors to relapses, nonadherence—partial or complete discontinuation of antipsychotics—is a primary risk factor, contributing to a 5-fold increase in relapse risk.3
As such, optimal antipsychotic selection, dosing, and monitoring play an important role in managing this illness. Patients with first-episode psychosis (FEP) are unusual in some ways, compared with patients with multiple episodes of psychosis and represent a different stage of schizophrenia.
In this 2-part series, we will discuss pharmacotherapy for FEP. This article focuses on antipsychotic selection, dosage, and duration of treatment among these patients. The second article, in the July 2015 issue, reviews the rationale and evidence for non-standard, first-line therapies, including long-acting injectable antipsychotics and clozapine.
Defining FEP
FEP refers to a patient who has presented, been evaluated, and received treatment for the first psychotic episode associated with a schizophrenia spectrum diagnosis.4 FEP is part of a trajectory marked by tran sitional periods. The patient transitions from being “healthy” to a prodromal state characterized by: (1) nonpsychotic behavioral disturbances such as depression or obsessive-compulsive disorder, (2) attenuated psychotic symptoms not requiring treatment, then converting to (3) psychotic symptoms prompting initial presentation for antipsychotic pharmacotherapy, leading to (4) a formal diagnosis of schizophreniform disorder and, subsequently, schizophrenia, requiring treatment to stabilize symptoms.
There are 2 critical periods along this continuum: prodromal stage and the duration of untreated psychosis (DUP). The prodromal period is a retrospectively identified time where the patient shows initial nonpsychotic disturbances (eg, cognitive and behavioral symptoms) before exhibiting clinical diagnostic criteria for a schizophrenia spectrum disorder. Approximately one-third of patients exhibiting these symptoms convert to psychosis within 1 year, and early treatment engagement at this stage has been shown to improve outcomes.5 The DUP is the time from when a patient has noticeable psychotic symptoms to initiation of drug treatment. The DUP is a consistent predictor of clinical outcome in schizophrenia, including negative symptoms, quality of life, and functional capacity.1
Antipsychotic selection
Treatment goals for FEP patients include:
• minimizing the DUP
• rapidly stabilizing psychosis
• achieving full symptomatic remission
• preventing relapse.
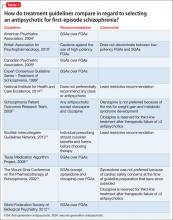
Several treatment guidelines for managing schizophrenia offer variable recommendations for initial antipsychotic treatment in patients with first-episode schizophrenia (Table 1).6-15 Most recommend second-generation antipsychotics (SGAs) over first-generation antipsychotics (FGAs)6,8,9,13,15 with specific recommendations on minimizing neurologic and metabolic adverse effects—to which FEP patients are susceptible—by avoiding high-potency and neurotoxic FGAs (eg, haloperidol and fluphenazine),7 clozapine,11,14 olanzapine,11 or ziprasidone.14 Two guidelines—the National Institute for Health and Care Excellence and the Scottish Intercollegiate Guidelines Network—do not state a preference for antipsychotic selection.10,12
The rationale for these recommendations is based on efficacy data, tolerability differences, FDA-approved indications, and recent FDA approvals with sparse post-marketing data. Of note, there are a lack of robust data for newer antipsychotics (eg, aripiprazole, paliperidone, iloperidone, asenapine, and lurasidone) in effectively and safely treating FEP; however, given the results of other antipsychotics studies, it is likely the efficacy and tolerability of these drugs can be extrapolated from experience with multi-episode patients.
Study design and demographics. Research studies of FEP share some similarities in study design; however, there is enough variability to make it difficult to compare studies and generalize findings (Table 2).16 The variability of DUP is a limitation when comparing studies because it is a significant predictor of clinical outcome. Patients who abuse substances—and often are more challenging to treat17—typically are excluded from these trials, which could explain the high response rate documented in studies of first-episode schizophrenia.
In addition, some FEP patients included in clinical trials might not be truly antipsychotic naïve; an estimated 25% to 75% of patients in these studies are antipsychotic naïve. This is an important consideration when comparing data on adverse effects that occur early in treatment. Additionally, acknowledging the advantages and disadvantages of how to handle missing data is critical because of the high dropout rate observed in these studies.18
Efficacy. There is a high response rate to antipsychotic therapy—ranging from 46% to 96%, depending on the study—in patients with first-episode schizophrenia.3 The response mainly is seen in reduction of positive symptoms because typically negative and cognitive symptoms do not respond to antipsychotics. One study reported only 29% of patients achieved both positive and negative symptom remission.19 It is likely that secondary negative symptoms caused by social withdrawal, reduced speech, and avoidance improve when positive symptoms subside, but primary negative symptoms endure.In general, there is a lack of evidence suggesting that 1 antipsychotic class or agent is more effective than another. Studies mainly assess effectiveness using the primary outcome measure of all-cause discontinuation, such as the Clinical Antipsychotic Trials of Intervention Effectiveness study.20 This outcome measure is a mixture of patient preference, tolerability, and efficacy that provides a more generalizable gauge on how well the treatment works in the clinic rather than tightly regulated settings such as clinical trials. A recent meta-analysis supports no differences in efficacy among antipsychotics in early-episode psychosis.21