Lisdexamfetamine, approved by the FDA in 2007 for attention-deficit/hyperactivity disorder (ADHD), has a new indication: binge eating disorder (BED) (Table 1). BED is characterized by recurrent episodes of consuming a large amount of food in a short time. A prodrug of amphetamine, lisdexamfetamine is a Schedule-II controlled substance, with a high potential for abuse and the risk of severe psychological or physical dependence.
Lisdexamfetamine is not indicated for weight loss or obesity.
Dosage
For BED, the initial dosage of lisdexamfetamine is 30 mg/d in the morning, titrated by 20 mg/d per week to the target dosage of 50 to 70 mg/d. Maximum dosage is 70 mg/d. Morning dosing is recommended to avoid sleep disturbance.
Efficacy
The clinical efficacy of lisdexamfetamine was assessed in two 12-week parallel group, flexible-dose, placebo-controlled trials in adults with BED (age 18 to 55). Primary efficacy measure was the number of binge days per week. Both studies had a 4-week dose-optimization period and an 8-week dose-maintenance period and followed the same dosage protocol. Patients began treatment at 30 mg/d and after 1 week were titrated to 50 mg/d; increases to 70 mg/d were made if clinically necessary and well tolerated. Patients were maintained on the optimized dosage during the 8-week dose-maintenance period. A dosage of 30 mg/d did not produce a statistically significant effect, but 50 mg/d and 70 mg/d dosages were statistically superior to placebo. Patients taking lisdexamfetamine also had greater improvement on the Clinical Global Impression—Improvement scores, 4-week binge cessation, and greater reduction in the Yale-Brown Obsessive Compulsive Scale Modified for Binge Eating score.
The prescribing information does not state if lisdexamfetamine should be continued long-term for treating BED.
Adverse reactions
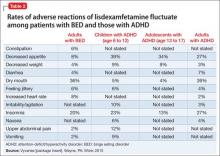
In controlled trials, 5.1% of patients receiving lisdexamfetamine for BED discontinued the drug because of an adverse event, compared with 2.4% of patients receiving placebo. The most common adverse reactions in BED studies were dry mouth (36%), insomnia (20%), decreased appetite (8%), increased heart rate (8%), constipation (6%), and feeling jittery (6%). In trials of children, adolescents, and adults with ADHD, decreased appetite was more common (39%, 34%, and 27%, respectively) than in BED trials (Table 2). Anaphylactic reactions, Stevens-Johnson syndrome, angioedema, and urticaria have been described in postmarketing reports.
The safety of lisdexamfetamine for BED has not been studied in patients age <18, but has been studied in patients with ADHD.
Contraindications
Do not give lisdexamfetamine to patients who have a known hypersensitivity to amphetamine products or other ingredients in lisdexamfetamine capsules.
Lisdexamfetamine is contraindicated in patients who are taking a monoamine oxidase inhibitor, because of a risk of hypertensive crisis.
Related Resources
• Wilens TE. Lisdexamfetamine for ADHD. Current Psychiatry. 2007;6(6):96-98,105.
• Peat CM, Brownley KA, Berkman ND, et al. Binge eating disorder: evidence-based treatments. Current Psychiatry. 2012; 11(5):32-39.